The cervix industry has had implants to prevent pregnancy since the late 1960s, but there hasn’t exactly been stiff competition to slow down the fallopian swim team at its source. In fact, Contraline claims it is the first major innovation in this space since the vasectomy was performed on a human some 125 years ago. The company calls its product ADAM, and it just raised a wad of cash to continue its trials.
“The first-in-human male contraceptive implant is a major clinical milestone that opens up new possibilities for men who wish to take contraception into their own hands,” said Kevin Eisenfrats, Co-founder and CEO of Contraline. “The patient demand for the ADAM Study has been tremendous, with the entire trial oversubscribing within three weeks of opening enrollment. We are looking forward to advancing ADAM through clinical development and bringing this product to market to transform how people think about contraception.”
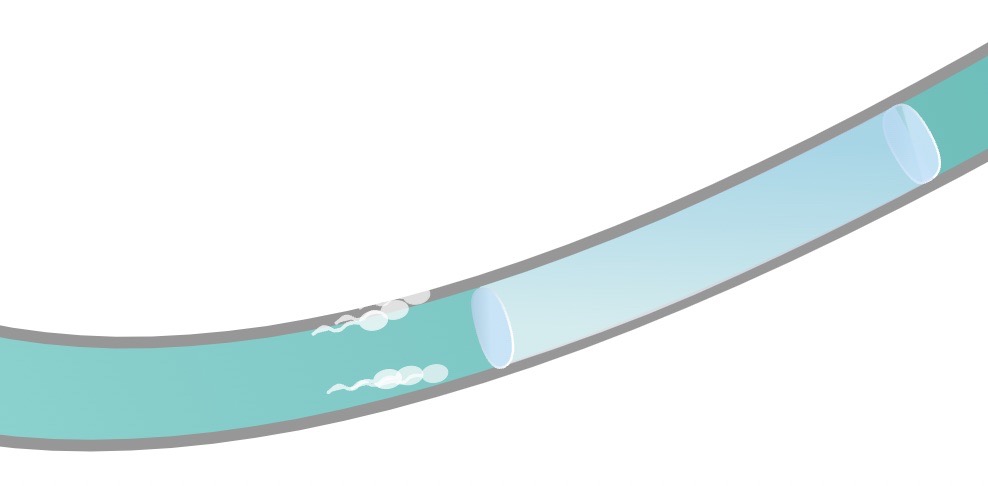
ADAM works by injecting a hydro gel into the vas deferens (the little tubes that carry the sperm). Image Credits: Contraline.
The company just raised $7.2 million in funding led by GV. The goal is to advance its in-human clinical trials of its injectable hydrogel designed to provide long-lasting, non-permanent contraception for men. The product uses a “hydrogel” designed to occlude sperm flow through the vas deferens for a predefined period of time, eventually degrading and thus offering a non-permanent contraceptive option.
The company suggests that the contraceptive is long-lasting but non-permanent, and claims it has no hormonal impact on the patients. The company told TechCrunch that four men were implanted with ADAM at a hospital in Australia, using a minimally invasive, no-scalpel approach, with ADAM being injected using a patent-pending delivery device.
The procedure marks the first patient implanted in “The ADAM Study,” which is being conducted under Human Research Ethics Committee approval. The ADAM Study is assessing the safety of the ADAM Hydrogel, while monitoring the semen parameters of the study subjects over three years.
“Contraline has the potential to fundamentally change the market for contraception,” said Cathy Friedman, executive venture partner at GV. “We look forward to working with the team as they continue developing a long-acting, reversible male contraceptive that empowers more people with more choices over family planning.”
Contraline’s study in Australia continues, and its next, longer-term goal is to run a second study with a larger group of patients in the United States.
Contraline erects $7.2M for contraceptive implants for men by Haje Jan Kamps originally published on TechCrunch
DUOS





